|
 |
| J Korean Soc Cosmetol > Volume 28(5); 2022 > Article |
|
Abstract
Although many plants have been investigated for their antifungal activity against candidiasis, more treatment options remain needed. This study evaluated the antifungal activity of 19 essential oils against Candida albicans. Nineteen essential oils, at concentrations of 1% and 0.5%, were added to cultures of C. albicans and incubated at 37°C for 18 h. The optical density was measured at 600 nm. The antifungal activity of the essential oils against C. albicans was dependent on concentration and the extraction site. At 0.5%, essential oils from thyme, palmarosa, and lemongrass displayed higher antifungal activities than fluconazole, suggesting that they are effective against C. albicans. The essential oils of eucalyptus, rosewood, and lavender showed the highest activity at 1% concentration and may be used as antibiotics individually. In addition, the essential oils extracted from leaves, such as thyme, were found to have higher antifungal activity than those from other sites.
Candida albicans is among the most prevalent fungal species of the human microbiota that asymptomatically colonizes healthy individuals (Lohse et al., 2018). These fungal infections can develop from simple skin mucosa infections to infections that can ultimately invade any organ (Pappas et al., 2004). Although antibiotics are widely used due to their high efficiency, strains resistant to these antibiotics are being discovered one after another, and the development of antibiotics with high efficiency is required (Mukeherjee et al., 2003).
Before the introduction of antibiotics and other modern drugs, natural products have been used globally for decades as traditional medicine. According to the World Health Organization (WHO), medicinal plants are the best source of various drugs (Khan et al., 2009). Essential oils (EOs), derived from the roots, stems, leaves, and flowers of different plants, are used in folk medicine as an alternative to modern medicines. In addition, they have been widely utilized in drug discovery and development, and they are readily accessible (da Silva et al., 2016; Myszka et al., 2016; Tutar et al., 2016). Recently, EOs have attracted significant interest owing to their antibacterial and antifungal activities, which are associated with their complex composition (Ghanem & Olama, 2014; Ceylan & Ugur, 2015). Several reports demonstrated the antifungal properties of extracts from different parts of various plants (Okla et al., 2021; Mutlu-Ingok et al., 2020). Clinical candidiasis has a steadily increasing annual infection rate (Tati et al., 2016). The effective antifungal and antibiofilm activities of several EOs against Candida spp. have been demonstrated (Abu-Darwish et al., 2016; Peixoto et al., 2017). Although there have been many studies on the antibiotic susceptibility of fungi to antifungal agents, only a few have reported on the susceptibility of C. albicans to EOs. This study conducted an antibacterial test by randomly selecting 19 types of well-known oils so that essential oils can be used in various practical applications.
C. albicans (ATCC 13316) was purchased from KCTC (Korea Collection for Type Cultures). The 19 EOs were randomly selected from the products supplied by the Korean Aromatherapy Certification Society. They were classified according to the extraction site (Table 1). Fluconazole (Sigma-Aldrich, Steinheim, Germany) was used as a control, following the CLSI standard amount (8 μg/ml).
C. albicans was plated on Blood agar (MB-N1036, Kisan Bio, Seoul, Korea), and a nutrient broth culture with 1 × 106 colony-forming units/mL was prepared using a 0.5 McFarland standard. After dispensing 100 μl of the bacterial suspension into a 96-well plate, 100 μl of essential oils (1% and 0.5% concentrations) or 100 μl of fluconazole (CLSI: Clinical and Laboratory Standards Institute standard concentration; 8 μg/ml) were added 3 each wells, and the plate was then incubated at 37°C for 24 h in an incubator. The essential oil concentrations of 1% and 0.5% were diluted using the nutrient broth (Difco, Le Pont-de-Claix, France). The essential oil diluent was homogenized by voltexing for at least 1 minute to properly mix with the medium. And pipeting was done at medium depth.
OD was detected at 600 nm as an endpoint using the spectrophotometer absorption program of the FlexStation 3 Multi-Mode Microplate Reader (V3.4.4.2413) (Molecular Devices, California, USA). OD reading was performed in four replicates to ensure the accuracy of the results.
For statistics, IBM SPSS Statistics 20 (IBM Corp., NY, USA) program was used, and a one-way ANOVA test was performed to verify the statistical significance between the control group and the experimental group. And the significance test was performed at the level of *P < 0.05, **P < 0.01, ***P < 0.001; #P < 0.05, ##P < 0.01. * = Significance compared to control, # = Significance compared to fluconazole. This experiment was repeated three times.
After reading each essential oil with a wavelength of 600 nm in a micro plate reader, the inhibition rate is shown in Table 2 and Figure 1, 2 by comparing the resulting value with the Control. First, compared to the control (100%), the inhibition rate of fluconazole was 50%. Lemon (75%), Thyme (74%), Lemongrass (72%), Cedarwood (68%), Cypress (66%), Rosemary (65%), Chamomile blue (62%), Jasmine (51%) at 0.5% Concentration showed higher antibacterial activity than fluconazole (50%) (Fig. 1). In particular, Lemon (75%), Thyme (74%) and Lemongrass (72%) showed the highest antibacterial activity. The inhibition rates observed in palmarosa respectively, showing the same inhibition rate as fluconazole (50%). However, the remaining ten essential oils had lower antibacterial activity than fluconazole. Even in the case of sandalwood, C. albicans grew even more.
At 1.0% concentration, Thyme (78%), Cypress (76%), Lemon (70%), Rose geranium (69%), Cedarwood atlas (66%), Palmarosa (66%), Jasmine (64%), Rosewood (64%), Lemongrass (63%), Rosemary verbenone (57%) had higher antimicrobial activity than fluconazole (72%) (Fig. 2). Thyme (78%), Cypress (76%), and Lemon (70%) had the highest antibacterial activity. Eucalyptus (50%) had similar values for fluconazole. However, the rest of the essential oils had lower antibacterial activity than fluconazole. In the case of sandalwood, the bacteria were more active.
To verify the significance of the following results, one-way ANOVA analysis was performed. The part showing significance at 0.5% compared to Control was marked with *. Palmarosa and patchouli showed the highest significance, and all oils except ylang-ylang, tea tree, sandalwood, cedarwood, cypress and lemon showed significance. At 1.0%, almost all essential oils showed high significance, and lemon and bergamot showed no significance. The portion showing significance at 0.5% compared to oxacillin was marked with #. Rosewood and bergamot showed the highest significance, and all oils except rosemary, ylang-ylang, manuka, orange and cedarwood showed significance. At 1.0%, significance was shown in the remaining essential oils except for thyme, ylang-ylang, manuka, cedarwood, tea tree, and rosewood.
In general, essential oils isolated from leaves showed higher antimicrobial activity than those isolated from other plant parts.
In order to compare and analyze the antibiotic effect of essential oils on C. albicans, a cause of fungal vaginosis, with fluconazole, an antibacterial test was conducted on 19 kinds of essential oils available in Korea. Previous reports indicated that the EOs of thyme, pennyroyal, and lemon inhibited the growth of various fungi, including C. albicans (Mahdavi & Esmaeilzadeh, 2009). Patchouli oil was effective against several gram-positive and gram-negative bacteria and fungal species, including C. albicans, although the findings were contradictory (Adhavan et al., 2017; Karimi, 2014; Li et al., 2012). In this study, LG, EC, and TM showed excellent antifungal effects against C. albicans. The antifungal activity of EC was also established in earlier studies (Kim et al., 2011) and observed to be comparable to that of fluconazole in our study.
We also confirmed that the antifungal activity of EOs against C. albicans was concentration-dependent, similar to findings from a previous study. Another study confirmed the growth inhibitory effect of Manuka oil (MK) on C. albicans at a concentration of 0.01% (Kim et al., 2013). On similar lines, the present study confirmed that the antifungal effect of MK was higher at 0.5% than at 1.0%. These results show that MK exhibits higher antifungal activity at lower concentrations. In Lee et al., 2012 paper, palmarosa showed strong antibacterial activity against C. albicans compared to geranium, which is consistent with our study results.
Moreover, we concluded that the antifungal activity of EOs differed based on the extraction site. EOs extracted from the leaves, particularly TM, had the highest antifungal activity. In previous papers, it was confirmed that essential oils of Lamiaceae and Camphoraceae plants with antibacterial activity had excellent antibacterial activity. These plants contain a lot of aldehyde and alcohol compounds (Yi and Bu, 2017). According to previous studies, TM induces changes in the enzyme profile of C. albicans. These changes lead to an inability to assimilate saccharides and, consequently, may significantly impact the pathogenicity of C. albicans (Rajkowska et al., 2014). TM also disrupted C. albicans biofilms (Alves et al., 2019). The antifungal activity of Lemongrass has already been reported several times and (Heral et al., 2006; Paranagama et al., 2003) activity was citral, such as limonene, citronellal, β-myrcene, linalool and geraniol, etc. It is known to be caused by several components (Rauber et al., 2005). In particular, according to Abe et al. (2003), the main component of Lemongrass is citral It inhibits Candida mycelia growth and yeast growth. It is known that antibacterial activity against this ingredient can be expected. In this regard, the antibacterial power of citral It can be explained by the same high as this Lemongrass was presumed to be. In addition, Rosewood and Coriander et al. linalool It is an essential oil of the alcholol family with It requires further analysis and consists of dozens of different components. The essential oil has a synergistic effect between the main ingredients and trace ingredients. It is expected to have an antibiotic effect by producing or inhibiting (Deba et al., 2007). LM was the most effective among the oils extracted from fruit peels. SW, BG, and YY did not exhibit antifungal activity against C. albicans, as suggested by earlier studies (Powers et al., 2018; Bona et al., 2016; Devkatte et al., 2005).
This study has several limitations. A crucial challenge was that only limited amounts of EOs at varying concentrations were available. Therefore, further studies using several other concentrations of EOs are required. Nevertheless, our study showed the antifungal activities of 19 different EOs compared to fluconazole. Since each EO showed distinct antifungal activity based on the concentration and some were more effective in a lower concentration, they may be used independently. Overall, this study presented useful preliminary data on the utilization of EOs against C. albicans.
Fig. 1.
Antifungal effect of 19 essential oils (0.5 %) against C. albicans. The antibacterial activities of the essential oils are arranged in ascending order from left to right. * = Significance compared to control, # = Significance compared to Fluconazole *P < 0.05, **P < 0.01, ***P < 0.001; #P < 0.05, ##P < 0.01
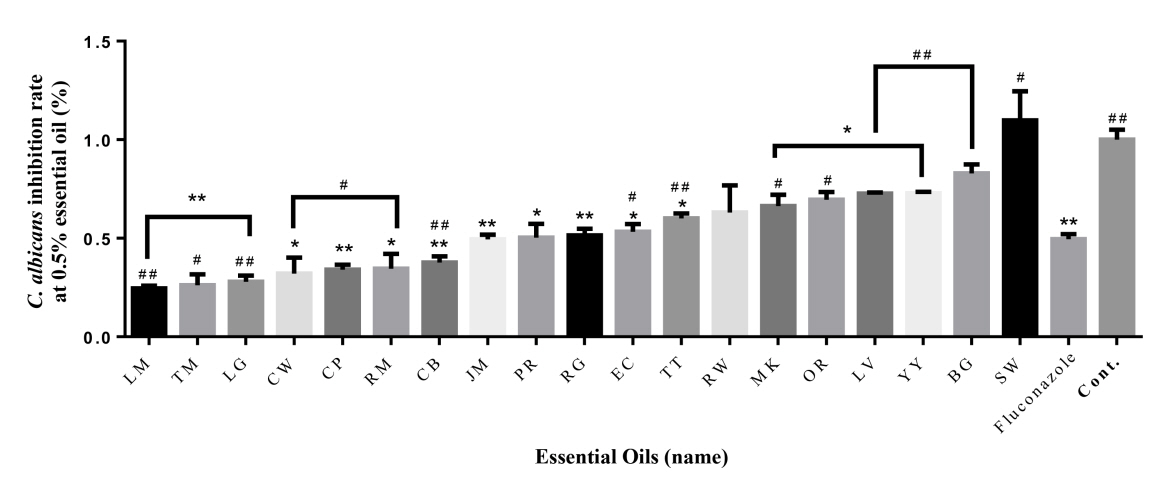
Fig. 2.
Antifungal effect of 19 essential oils (1%) against C. albicans. The antibacterial activities of the essential oils are arranged in ascending order from left to right. * = Significance compared to control, # = Significance compared to Fluconazole *P < 0.05, **P < 0.01, ***P < 0.001; #P < 0.05, ##P < 0.01
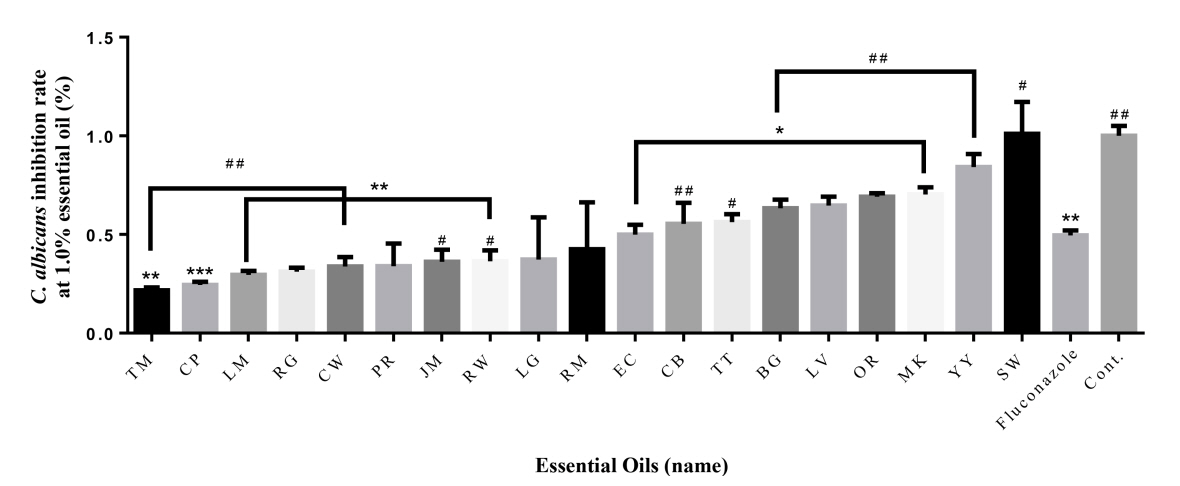
Table 1.
List of the essential oils used in this study and their extraction sites
Table 2.
Evaluation of the antifungal activity of the essential oils at 0.5 and 1.0% concentration
References
Abe, S., Sato, Y., Inoue, S., Ishibashi, H., Maruyama, N., Takizawa, T., Oshima, H., & Yamaguchi, H. (2003). Anti-Candida albicans activity of essential oils including Lemongrass (Cymbopogon citratus) oil and its component, citral. Nihon Ishinkin Gakkai Zasshi, 44(4), 285-291.


Abu-Darwish, M. S., Cabral, C., Gonçalves, M. J., Cavaleiro, C., Cruz, M. T., Zulfiqar, A., Khan, I. A., Efferth, T., & Salgueiro, L. (2016 Chemical composition and biological activities of Artemisia judaica essential oil from southern desert of Jordan. J Ethnopharmacol, 191:161-168. https://doi.org/10.1016/j.jep.2016.06.023.


Adhavan, P., Kaur, G., Princy, A., & Murugan, R. (2017 Essential oil nanoemulsions of wild patchouli attenuate multi-drug resistant gram-positive, gram-negative and Candida albicans. Ind Crops Prod, 100:106-116. https://doi.org/10.1016/j.indcrop.2017.02.015.

Alves, M., Gonçalves, M. J., Zuzarte, M., Alves-Silva, J. M., Cavaleiro, C., Cruz, M. T., & Salgueiro, L. (2019 Unveiling the antifungal potential of two Iberian thyme essential oils: effect on C. albicans germ tube and preformed biofilms. Front Pharmacol, 10:446.https://doi.org/10.3389/fphar.2019.00446.

Bona, E., Cantamessa, S., Pavan, M., Novello, G., Massa, N., Rocchetti, A., Berta, G., & Gamalero, E. (2016 Sensitivity of Candida albicans to essential oils: are they an alternative to antifungal agents? J Appl Microbiol, 121(6), 1530-1545. https://doi.org/10.1111/jam.13282.


Ceylan, O., & Ugur, A. (2015 Chemical composition and anti-biofilm activity of Thymus sipyleus BOISS. Subsp. sipyleus BOISS. Var. davisianus RONNIGER essential oil. Arch Pharm Res, 38:957-965. https://doi.org/10.1007/s12272-014-0516-0.

Sant'Anna Da Silva, A. P., Nascimento da Silva, L. C., Martins da Fonseca, C. S., de Araújo, J. M., & dos Santos Correia, M. T. (2016 Antimicrobial activity and phytochemical analysis of organic extracts from Cleome spinosa Jaqc. Front Microbiol, 7:963.https://doi.org/10.3389/fmicb.2016.00963.



Deba, F., Xuan, T. D., Yasuda, M., & Tawata, S. (2007). Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control, 19(4), 346-352.
Devkatte, A. N., Zore, G. B., & Karuppayil, S. M. (2005 Potential of plant oils as inhibitors of Candida albicans growth. FEMS Yeast Res, 5(9), 867-873. https://doi.org/10.1016/j.femsyr.2005.02.003.


Ghanem, S., & Olama, Z. (2014). Antimicrobial potential of Lebanese cedar extract against human pathogens and food spoilage microorganisms. EJBPSP, 1:13-26.
Heral, G. A., Sarhan, M. M., Abu Shahala, A. N., & Abou El-Khair, E. K. (2006). Effect of Cymbopogon citratus L. essential oil on growth and morphogenesis of Saccharomyces cerevisiae ML2- strain. J Basic Microbiol, 46(5), 375-386.


Karimi, A. (2014). Characterization and antimicrobial activity of patchouli essential oil extracted from Pogostemon cablin [Blanco] Benth. Adv Environ Biol, 8:2301-2309.
Khan, R., Islam, B., Akram, M., Shakil, S., Ahmad, A., Ali, S. M., Siddiqui, M., & Khan, A. U. (2009 Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules, 14(2), 586-597. https://doi.org/10.3390/molecules14020586.



Kim, H.-S., Lee, H.-Y., Lee, J.-N., Joo, C.-G., & and Choe, T.-B. (2011). The Effects of Antimicrobial Properties of Manuka Oil and Improvement of Acne. Journal of the Korean Society of Cosmetology, 17(2), 245-256.
Kim, J.-H., Kim, M.-J., Choi, S.-K., Bae, S.-H., An, S.-K., & Yoon, Y.-M. (2011). Antioxidant and antimicrobial effects of lemon and eucalyptus essential oils against skin floras. J Soc Cosmet Sci Korea, 37:303-308.
Lee, E.-J., Lee, S.-H., & Lim, M.-H. (2012). Antimicrobial and Antioxidative Activities Essential Oil-focused on Palmarosa (Cymbopogen marini) and Geranium (Pelargonium graveolens). Journal of the Korean Society of Cosmetology, 18(1), 136-143.
Li, Y. C., Liang, H. C., Chen, H. M., Tan, L. R., Yi, Y. Y., Qin, Z., Zhang, W.-M., Wu, D.-W., Li, C.-W., Lin, R.-F., Su, Z.-R., & Lai, X.-P. (2012 Anti-Candida albicans activity and pharmacokinetics of pogostone isolated from Pogostemonis Herba. Phytomedicine, 20(1), 77-83. https://doi.org/10.1016/j.phymed.2012.08.008.


Lohse, M. B., Gulati, M., Johnson, A. D., & Nobile, C. J. (2018 Development and regulation of single- and multi-species Candida albicans biofilms. Nat Rev Microbiol, 16:19-31. https://doi.org/10.1038/nrmicro.2017.107.


Mahdavi, O. S., & Esmaeilzadeh, S. (2009). Comparison of anti-candida activity of thyme, pennyroyal, and lemon essential oils versus antifungal drugs against candida species. Jundishapur J Microbiol, 2:53-60.
Mukherjee, P. K., Chandra, J., Kuhn, D. M., & Ghannoum, M. A. (2003). Mechanism of Fluconazole Resistance in Candida albicans Biofilms: Phase-Specific Role of Efflux Pumps and Membrane Sterols. Infect Immun, 71(8), 4333-4343.




Mutlu-Ingok, A., Devecioglu, D., Dikmetas, D. N., Karbancioglu-Guler, F., & Capanoglu, E. (2020 Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: an updated review. Molecules, 25(20), 4711.https://doi.org/10.3390/molecules25204711.



Myszka, K., Schmidt, M. T., Majcher, M., Juzwa, W., Olkowicz, M., & Czaczyk, K. (2016 Inhibition of quorum sensing-related biofilm of Pseudomonas fluorescens KM121 by Thymus vulgare essential oil and its major bioactive compounds. Int Biodeterior Biodegrad, 114:252-259. https://doi.org/10.1016/j.ibiod.2016.07.006.

Okla, M. K., Alatar, A. A., Al-Amri, S. S., Soufan, W. H., Ahmad, A., & Abdel-Maksoud, M. A. (2021 Antibacterial and antifungal activity of the extracts of different parts of Avicennia marina (Forssk.) Vierh. Plants (Basel), 10(2), 252.https://doi.org/10.3390/plants10020252.



Pappas, P. G., Rex, J. H., Sobel, J. D., Filler, S. G., Dismukes, W. E., Walsh, T. J., & Edwards, J. E. (2004). Guidelines for Treatment of Candidiasis. Clin Infect Dis, 38(2), 161-189.



Paranagama, P. A., Abeysekera, K. H. T., Abeywickrama, K., & Nugaliyadde, L. (2003). Fungicidal and anti-aflatoxigenic effects of the essential oil of Cymbopogon citratus (DC.) Stapf. (lemongrass) against Aspergillus flavus Link. isolated from stored rice. Lett Appl Microbiol, 37(1), 86-90.


Peixoto, L. R., Rosalen, P. L., Ferreira, G. L., Freires, I. A., de Carvalho, F. G., Castellano, L. R., & Castro, R. D. (2017). Antifungal activity, mode of action and anti-biofilm effects of Laurus nobilis Linnaeus essential oil against Candida spp. Arch Oral Biol, 73:179-185.


Powers, C. N., Osier, J. L., McFeeters, R. L., Brazell, C. B., Olsen, E. L., Moriarity, D. M., Satyal, P., & Setzer, W. N. (2018). Antifungal and cytotoxic activities of sixty commercially available essential oils. Molecules, 23(7), 1549.



Rajkowska, K., Kunicka-Styczyńska, A., Maroszyńska, M., & Dąbrowska, M. (2014 The effect of thyme and tea tree oils on morphology and metabolism of Candida albicans. Acta Biochim Pol, 61(2), 305-310. https://doi.org/10.18388/abp.2014_1900.


Rauber, C da S., Guterres, S. S., & Schapoval, E. E. (2005). LC determination of citral in Cymbopogon citratus volatile oil. J Pharm Biomed Anal, 37(3), 597-601.


Silva, Cde B., Guterres, S. S., Weisheimer, V., & Schapoval, E. E. (2008). Antifungal activity of the lemongrass oil and citral against Candida spp. Braz J Infect Dis, 12(1), 63-66.


Tati, S., Davidow, P., McCall, A., Hwang-Wong, E., Rojas, I. G., Cormack, B., & Edgerton, M. (2016). Candida glabrata binding to Candida albicans hyphae enables its development in oropharyngeal candidiasis. PLOS Pathog, 12:e1005522.



Tutar, U., Çelik, C., Karaman, İ., Ataş, M., & Hepokur, C. (2016). Anti-biofilm and antimicrobial activity of Mentha pulegium L. essential oil against multidrug-resistant Acinetobacter baumannii. Trop J Pharm Res, 15(5), 1039-1046.

Yi, M.-R., & Bu, H.-J. (2017). Antioxidant, Antimicrobial and Melanogenesis Inhibition Effects of 35 species Essential Oil. Journal of the Korean Society of Cosmetology, 23(4), 677-687.
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 874 View
- 13 Download
- Related articles
-
Antibiotic Effect of Essential Oils on Candida albicans2016 December;22(6)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print